Pharmacovigilance automation built for modern life sciences. Screver AI continuously scans feedback, forms, and digital touchpoints for potential adverse events – alerting your PV teams instantly, with full traceability and audit-ready logs.




















Screver Pharmacovigilance solution integrates seamlessly into your existing stack – websites, call centers, CRMs, and digital channels – to monitor safety signals in real time. Our AI agent detects and classifies potential adverse events across unstructured text, validates context using regulatory-trained models, and routes verified alerts to the responsible teams.
Understands medical, therapeutic, and regulatory terminology with pharma-trained AI models.

Monitors safety-related mentions across websites, surveys, portals, CRMs, and call logs in one unified workflow.

Every event is logged with full audit history and automated report generation for internal or external safety teams.

Screver monitors unstructured feedback across every touchpoint – from websites, surveys, and call transcripts to CRMs and chatbots.
It continuously listens for spontaneous mentions of side effects or safety concerns, turning raw text into structured, compliant data streams ready for pharmacovigilance review.
Every company, therapy area, and product has its own terminology.
That’s why Screver RAG-based AI is trained on your Brand IQ – integrating your products list, safety keywords, and internal documentation.
The system uses this context to distinguish between relevant and irrelevant mentions, reducing false positives and increasing accuracy over time.

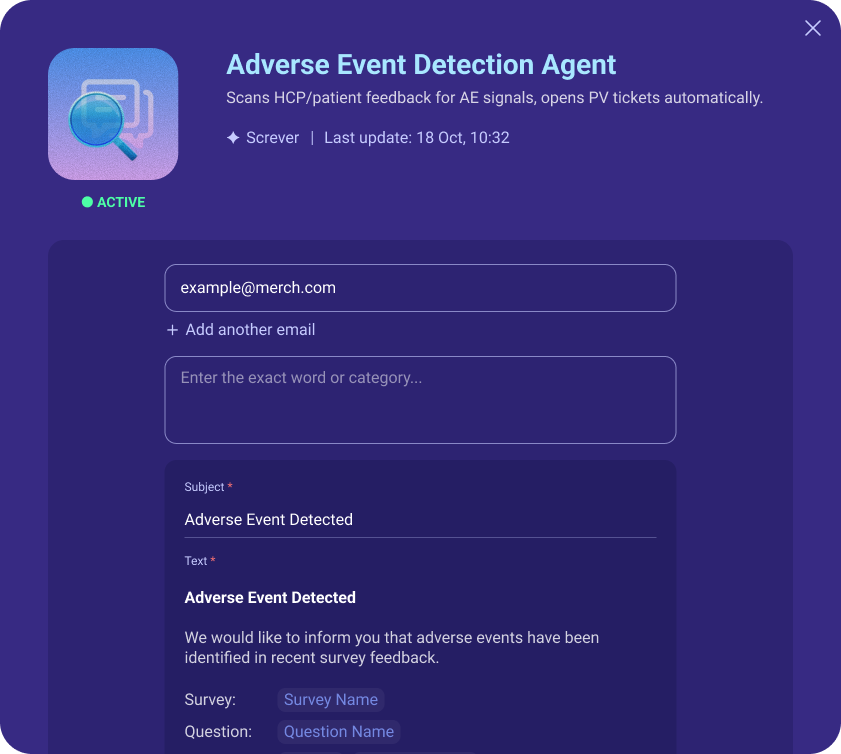
Once a potential event is confirmed, Screver automatically notifies the right pharmacovigilance or local safety team.
Reports can be triggered instantly or consolidated into daily or weekly safety summaries, with full traceability and audit logs.


We start with your reality, not a predefined model.
Every pharmacovigilance automation program begins with a focused pilot – typically for one product, one language, and one digital channel.
This lets us validate detection accuracy, routing logic, and compliance requirements before expanding across brands or regions.
Before launch, we collect essential reference materials – product guidelines, approved term lists, medical lexicons, and safety reporting SOPs – to add the context to our domain model and RAG layer.



Discover how Screver’s AI and RAG-based pharmacovigilance engine can monitor your websites, surveys, and digital platforms for potential safety events.